SARS-CoV-2 IgG Antibody Test
- This blood test checks for immunoglobulin G (IgG), an antibody that is produced by the body around 10 to 18 days after exposure to COVID-19 infection or after vaccination.
- Antibody production starts at around 5 days post-infection and reaches peak concentrations at 2 weeks for IgM and 3 weeks for IgG.
- This assay is intended for the quantitative detection of anti-SARS-CoV-2 IgG antibodies from serum, plasma, or fingertip blood samples.
- Targets the spike protein 1 (S1) RBD in the SARS-CoV-2 genome.
FDA EUA Filed I CE/IVD MARKED
Validated in CLIA-certified laboratory as an LDT
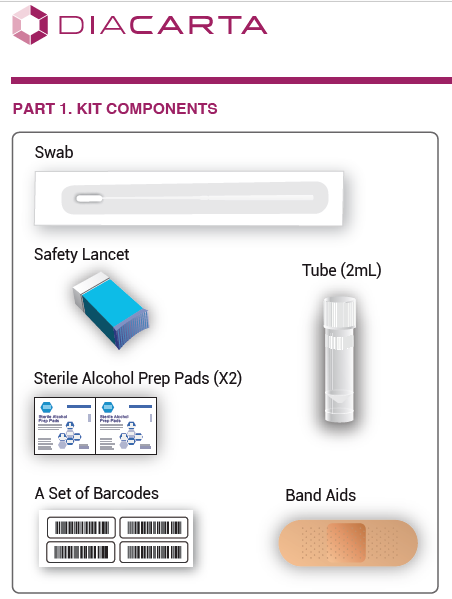
KIT COMPONENTS
STEP BY STEP
Separated they live in Bookmarksgrove right at the coast of the Semantics,
a large language ocean.
01 IDEA
Claritas est etiam processus dynamicus, qui sequitur mutationem consuetudium lectorum.
02 CONCEPT
Claritas est etiam processus dynamicus, qui sequitur mutationem consuetudium lectorum.
03 DESIGN
Claritas est etiam processus dynamicus, qui sequitur mutationem consuetudium lectorum.
04 DEVELOP
Claritas est etiam processus dynamicus, qui sequitur mutationem consuetudium lectorum.
05 TEST
Claritas est etiam processus dynamicus, qui sequitur mutationem consuetudium lectorum.